Stay Up to Date With
Our Vaccine Management Software
Trusted by EHR vendors, pharmacies, laboratories, hospitals, long-term care, and healthcare organizations nationwide, we seamlessly connect vaccine data with state registries, ensuring compliance and empowering our clients to access historical vaccine records and patient forecasting data. Discover new vaccine opportunities with ease.
64
Vaccine Registry Connections
Pub Hub offers nationwide access to vaccine records.
1.7M+ daily
Patients Searched
Pub Hub handles over 1.7 million vaccine history queries daily, ensuring timely, accurate information for healthcare providers and patients.
1M+ daily
Vaccines Submitted
Pub Hub processes and distributes over 1 million administered vaccine records daily to all 64 U.S. state and territorial vaccine registries.
An Intuitive, Streamlined
Enrollment Process
Our exclusive enrollment process offers a swift and simplified path for facilities to connect with state vaccine registries. With just a few steps—adding facilities, selecting registries, and completing paperwork—organizations can quickly onboard their providers and locations at scale, ensuring a hassle-free experience from start to finish.
Submit Immunizations and Query for your Patients' Data
Streamline your vaccine management with seamless integration to
64 state registries and proactive patient vaccination opportunities identification.
Discrete Vaccine Service
The discrete vaccine service is the fastest, most self-service method for submitting vaccine data to the state.
curl --location 'https://api.ironbridge.io/api/ph/discrete/vaccine/validate' \
--header 'Content-Type: application/json' \
--header 'Authorization: Bearer "Your Token" \
--data-raw '{
"version": "2.4",
"patient": {
"data_sharing": {
"protect_data": "N",
"date_of_consent": "20211015"
},
{ ... }
},
"vaccine_administration": [{
"vaccine": {
"code": "158",
"system": "CVX",
"description": "Influenza, injectable, quadrivalent (4576919)"
},
"completion_status": "CP",
"substance_lot_number": "1311-A",
{ ... }
}]
}'The discrete vaccine service is designed to accept a codified JSON payload for seamless integration. Featuring dynamic, state-specific validation, our service ensures compliance with state standards.
- State-Specific Validation. Our discrete vaccine service offers dynamic, state-specific compliance with state standards.
- Codified Error Payloads offering highly detailed error responses for precise troubleshooting. Each response provides codified details on the specific validation that failed, enabling swift identification and resolution of issues for seamless data integration.
- Webhooks for Updates Subscribe to the webhooks service to get status updates on submitted immunizations.
- Comprehensive Vaccine Codeset Maintenance Whether you submit CVX or NDC codes we have you covered. Our service maintains all vaccine related codesets in regards to the patient and the vaccine being administered.
Already have a data model? No problem.
While the discrete vaccine service allows you to validate your immunization data synchronously against the various state IIS systems, it's not the only method for submitting vaccines through the Iron Bridge Platform. If you already have a data model and want to work with that we have you covered. Contact us today to discuss options that work for you.
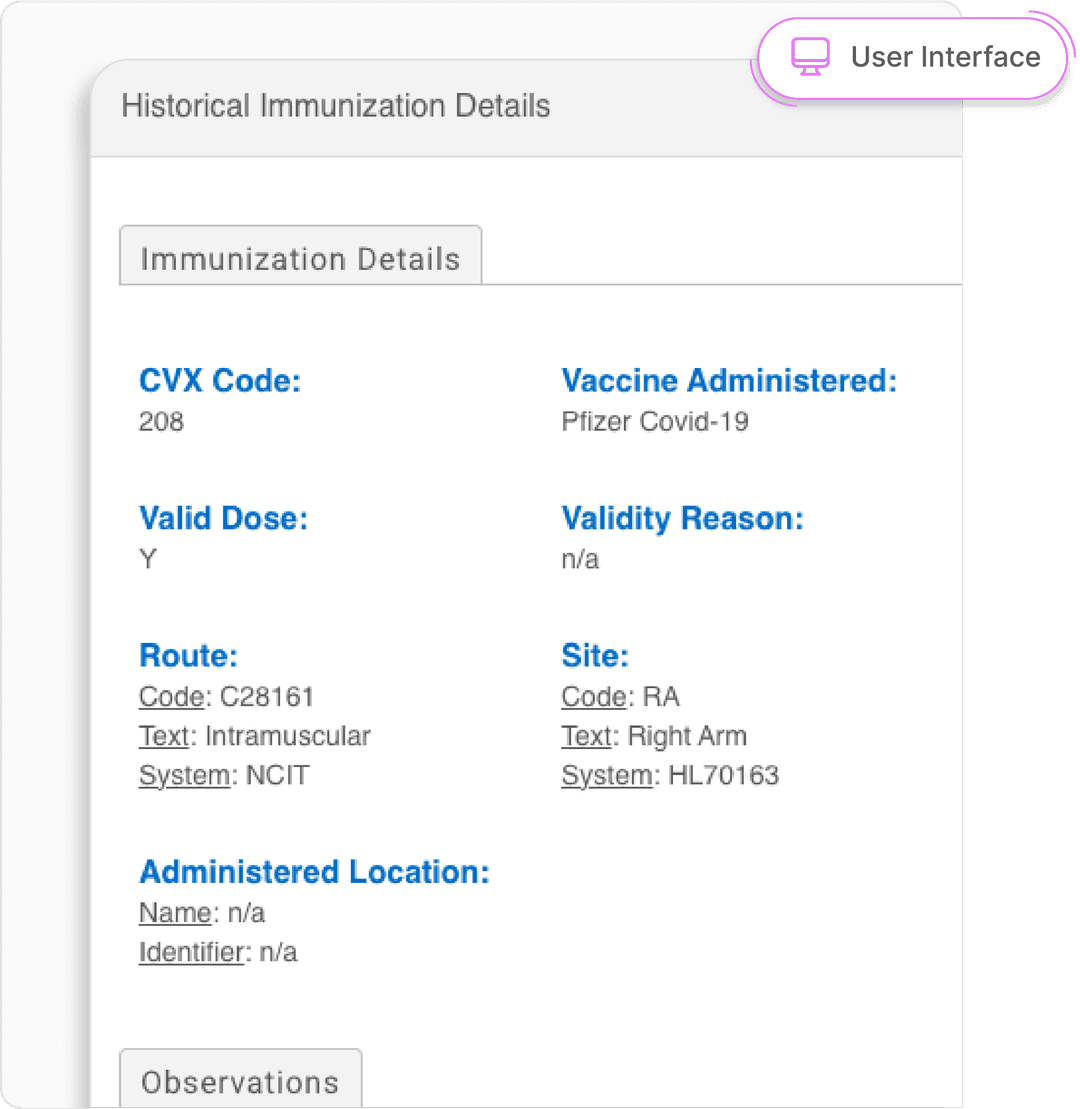
Retrieving a Patient's Historical Immunization and Forecasting Data
Iron Bridge makes your patient's vaccine history and forecasting data more accessible than ever with connections to 64 state registries.
- Immunization History
- Access and reconcile comprehensive immunization history from IIS into your patent records.
- Forecasting
- Receive vaccine recommendations based on patient’s age, immunization history, and ACIP vaccine schedule which allows querying entity to identify future vaccine opportunities.
- Seamless System Integration
- Integrate the patient query API directly into your patient chart or into existing vaccine workflows.
Multiple Ways to Query
The Pub Hub Vaccine Management Platform offers multiple methods to query for a patient's historical vaccine records and their vaccine forecasting data. Use one method or a combination of methods in order to get the data you need to make decisions.
- Real-time Synchronous API Service
Experience seamless integration and real-time patient data exchange within your application or existing workflows.
- User Interface Powered Query
Utilize the user-friendly patient query interface to navigate your patient's data effortlessly without any development efforts.
- Batch Query Process
Exchange batched patient demographic files based on patient appointment or scheduling data to proactively query for your patient's vaccine data.
Advanced Insights
Learn how Iron Bridge is ahead of the curve with ONC's HTI-1 Final Rule utilizing our multi tenant saas architecture in order to provide data insights that align with the certification requirements.


See what Iron Bridge has to offer.
Contact us today and schedule a demo to see how our products can help your company thrive.